Biochemistry:
Essential Skills
Essential Skills
Introduction
For each entry in the Topics list, here are a few of the most important skills you should develop before moving to the next topic. These lists include skills only, and do not include factual information that should be at your grasp are a result of reading the chapters and working problems.
View these as the absolutely essential skills that you need to possess in order to understand upcoming topics.
For help with electron pushing, look up
mechanisms of reactions in your organic textbook.
Introduction
Essential Skills
At the exam covering this chapter, you should be able to
- Distinguish structures of the four main classes of biomolecules: carbohydrates, lipids, nucleic acids, and proteins.
- Use the Gibbs equation,
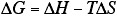 ,
,
to interrelate free-energy changes, enthalpy changes, and entropy changes for simple chemical and physical processes. - Know how free-energy changes for reactions are related to a) concentrations of reactants and products, and b) equilibrium constants.
- List and briefly describe the functions of the main organelles in a eucaryotic cell, and give the locations of major cellular processes, such as replication, energy production, and so forth.
Water
Essential Skills
At the exam covering this chapter, you should be able to
- Predict the noncovalent interactions between common organic functional groups.
- For weak acids HA, use the Henderson-Hasselbalch equation,
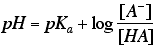 ,
,
to interrelate pH, pKa, [HA], [H+ ], and [A- ] in buffer solutions. - Draw or interpret titration curves for monoprotic and polyprotic acids.
- Use a table of pKa values to pick components for a buffer of specified pH.
- Push electrons to show the mechanisms of acid-base reactions.
Amino Acids and Protein Sequence
Essential Skills
This chapter brings you a large number of new skills that you will use througout your study of biochemistry.
At the exam covering this chapter, you should be able to
- Draw the structure of the 20 common amino acids, indicating proper ionization states at pH 7.0. Your instructor and the authors of your textbook will assume from this time forward that you have command of this information.
- Using a table of pKa values, determine the net charge of any amino acid or polypeptide at any pH, and estimate its pI.
- Determine whether a provided amino-acid model is D- or L-.
- Assemble peptide chains of specified sequence from memorized common amino-acid structures, or from uncommon amino acids, if provided.
- Given the molecular mass, pI, sequence, or other properties of proteins, predict their order of elution under these separation methods: ion-exchange chromatography, electrophoresis, hydrophobic-interaction chromatography, gel-filtration chromatography, SDS electrophoresis, ultracentrifugation, and affinity chromatography.
- Use a table of properties of partial hydrolysis reagents to a) predict cleavage points in polypeptides of specified sequence, and b) determine the sequence of a polypeptide from the sequences of partial hydrolysis products.
- Interpret phylogenetic trees to determine the relatedness or order of appearance of various organisms or proteins.
- Use amino-acid sequence comparisons of homologous proteins to find residues that might be important to structure or function.
- Push electrons to show the mechanisms of amide (= peptide) synthesis and hydrolysis.
- Push electrons to show the mechanism of the dansyl chloride reaction (structure of reagent provided).
- Push electrons to show the mechanism of the disulfide cleavage by mercaptoethanol (structure of reagent provided).
- Push electrons to show the mechanism of the Edman degradation (structure of reagent provided).
Structure and Function of Proteins
Essential Skills
At the exam covering this chapter, you should be able to
- Draw polypeptides realistically (Lewis wedge and dot), indicating atoms in and out of the plane of the peptide bond.
- Recognize common elements of secondary structure in perspective drawings of proteins, and in computer-graphics models.
- Determine from perspective drawings the handedness of coiled structures, such as helices, and of beta-sheet twists.
- Use the Gibbs equation to analyze protein folding.
- Give Deep View commands to accomplish basic selection, display, and coloring of computer protein models (see Sections 1-6 of Deep View Tutorial).
- Interpret, in molecular terms, saturation curves for simple (e.g., myoglobin) and cooperative (e.g., hemoglobin) ligand-binding proteins.
- Compute saturation fraction (Y) and transport efficiency (DY) for ligand-binding proteins.
- Predict the effects of changes in ligand or effector concentations on protein-ligand binding, formulate molecular explanations of these effects, and relate these effects to protein function.
- Push electrons to show the mechanism of action of protein disulfide isomerase.
Enzyme Kinetics
Essential Skills
At the exam covering this chapter, you should be able to
- Use the Michaelis-Menten equation,
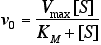
to compute enzymatic reaction rates under various conditions. - Construct and use double-reciprocal plots of kinetic data (concentrations of substrates or products versus time) to determine Km, Vmax, and kcat for Michaelis-Menten enzymes.
- Use double-reciprocal plots to distinguish competetive, noncompetitive, and uncompetitive inhibition, and to determine inhibitor dissociation constants (KI), using a reference like this Summary of Kinetic Effects of Reversible Inhibitors.
- Push electrons to sketch a general mechanism for the reaction catalyzed by phosphofructokinase.
Enzyme Mechanisms
Essential Skills
At the exam covering this chapter, you should be able to
- Predict the products of reactions catalyzed by triose phosphate isomerase, lysozyme, and serine proteases on normal substrates or on analogous hypothetical substrates.
- Recognize the various chemical modes and binding modes of catalytic mechanisms found in enzymatic reactions.
- From a proposed enzymatic mechanism, propose a structure for a transition-state analog that might inhibit the enzyme.
- Show the fate of labeled atoms in enzymatic reactions discussed in this chapter.
- Push electrons to show the mechanisms of action of triose phosphate isomerase, lysozyme, and serine proteases, given a diagram of catalytic groups and the structures of substrates.
Coenzymes and Vitamins
Essential
Skills
At the exam covering this chapter, you should be able to
- Recognize the reactive centers of coenzymes (from structures provided).
- Recognize the coenzymes likely to be involved in enzyme-catalyzed reactions (from reactants and products provided).
- Push electrons to show the mechanisms of coenzyme-mediated reactions (structures of all components provided).
Carbohydrates
Essential Skills
At the exam covering this chapter, you should be able to
- Distinguish enantiomers, diastereoisomers, epimers, and anomers of monosaccharides.
- Interpret and interconvert open-chain (Fisher projection) and ring (Haworth projection, furanose and pyranose) drawings of monosaccharides.
- Assemble oligosaccharides and glycopeptides from provided structures and descriptions of linkages (for example, a-1,4 links).
- Determine the hydrolysis products of oligo- or polysaccharides.
- Push electrons to show the mechanisms of hemiacetal/acetal and hemiketal/ketal formation and lysis in mono- and disaccharides.
Lipids and Membranes
Essential Skills
At the exam covering this chapter, you should be able to
- Predict the relative melting points of fatty acids and trigycerides.
- Assemble complex lipids (triacylglycerols, glycerphospholipids, sphingolipids, and lipopeptides) from descriptions of linkages and components provided.
- Determine the hydrolysis products of complex lipids.
- Predict the effects of changes in lipid composition on the phase-transition temperature and fluidity of lipid bilayers.
- Determine the orientation of vectorial membrane components after a series of membrane-fusion events.
- Calculate free-energy changes associated with transmembrane concentration and voltage gradients.
- Assess effects of changes in ligand and effector concentrations on transport systems, and signaling systems.
- Push electrons to show the mechanisms of ester and phosphate-ester hydrolysis.
Introduction to Metabolism
Essential Skills
Recommendation: If your text includes reduction potentials in this chapter, delay that topic until the chapter on electron transport.
At the exam covering this chapter, you should be able to
- Calculate one from the other: a) the free-energy change for a chemical reaction, and 2) the equilibrium constant or equilibrium concentrations of reactants and products.
- In equilibrium-constant expressions, use the proper biological standard-state conventions for water, hydrogen ion, solutes, and gases. See Plugging the Right Numbers Into Energy Calculations.
- Compute free-energy changes for metabolic reactions under standard
and cellular conditions.
- Formulate molecular explanations for the relative magnitudes of free energies of hydrolysis of so-called high energy compounds like ATP, based on changes in resonance stabilization, internal electrostatic interactions, solvation, and entropy.
- Compute free-energy changes for coupled reactions.
- Push electrons to show the mechanisms of kinase reactions.
Glycolysis
Essential Skills
- Apply all Essential Skills for Metabolic Pathways (list follows).
METABOLISM: Essential Skills for ALL Metabolic Pathways
Following are essential skills that apply to all metabolic pathways. Additional skills for individual chapters are provided after this list.
- Materials Flow: Trace the fate of labeled carbon or other elements through the pathway.
- Energy Flow: Trace the production and consumption of "high energy" compounds like ATP.
- Electron Flow: Trace the production and consumption of reducing power.
- Net Flows: Compute a) free-energy changes, b) net gain or loss of ATP, and c) net redox change during all of any segment of the pathway.
- Regulation: Explain the effects of allosteric effectors, hormones, second messengers, and reversible phosphorylation on individual enzymes and on overall flow through the pathway.
- Connections: Describe connections to other pathways.
- Push electrons to show the mechanisms of all pathway reactions (pathway diagrams with all structures of intermediates and cofactors provided on exams).
More Carbohydrate Metabolism
Essential Skills
- Apply all Essential Skills for Metabolic Pathways.
- Explain the function of substrate cycles in regulation.
- Use diagrams of regulatory cascades (such as Figures 12.16 and 12.17) to explain and predict the results of changing cellular conditions on metabolism.
Citric-Acid Cycle
Essential Skills
At the exam covering this chapter, you should be able to
- Apply all Essential Skills for Metabolic Pathways
Electron Transport and Oxidative
Phosphorylation
Essential Skills
At the exam covering this chapter, you should be able to
- Apply all Essential Skills for Metabolic Pathways
- Use tabulated reduction potentials to assess free-energy changes during stages of mitochondrial electron transport.
- Compute free-energy changes associated with establishment of proton and voltage gradients, and the use of these gradients as energy sources for ATP production by ATP synthase (F1FO-ATPase).
- Push electrons to show the mechanisms of reversible redox reactions of the following redox couples: NAD+/NADH with H:–, FAD/FADH2(with 2H. or with H:- + H+), and CoQ/CoQH2 (with 2H. or with H:- + H+).
Photosynthesis
Essential Skills
At the exam covering this chapter, you should be able to
- Apply all Essential Skills for Metabolic Pathways
- Explain, using electronic energy-level diagrams, the mechanisms by which light is absorbed by photosynthetic pigments, and by which excitation energy is transferred among pigments.
- Distinguish between excitation-energy transfer and electron transport.
- Use tabulated reduction potentials to assess free-energy changes during stages of chloroplast electron transport.
- Compute free-energy changes associated with establishment of proton and voltage gradients, and the use of these gradients as energy sources for ATP production by ATP synthase.
Lipid Metabolism
Essential Skills
At the exam covering this chapter, you should be able to
- Apply all Essential Skills for Metabolic Pathways
Amino-Acid Metabolism
Essential Skills
At the exam covering this chapter, you should be able to
- Apply all Essential Skills for Metabolic Pathways
Nucleotide Metabolism
Essential Skills
At the exam covering this chapter, you should be able to
- Apply all Essential Skills for Metabolic Pathways
Nucleic Acids
Essential Skills
At the exam covering this chapter, you should be able to
- Draw the base pairs A-T, A-U, and G-C with hydrogen bonds.
- Assemble bases, sugars, and phosphates into drawings of specified ribo- and deoxyribonucleotides and nucleic acids, using uncommon bases if provided.
- Describe the roles of DNA, mRNA, and proteins in the central dogma of molecular biology.
- Use the linking-number equation, L = T + W, to predict the
effects of over- or under-winding DNA, and the effects of topoisomerases
on DNA twist and supertwist.
This reading might help. - Push electrons to show the mechanism of phosphate-ester synthesis and hydrolysis.
- Push electrons to show the mechanism of alkaline hydrolysis of RNA and of RNA cleavage by RNase A.
Replication and Repair of DNA
Essential
Skills
At the exam covering this chapter, you should be able to
- Explain factors that contribute to fidelity of replication.
- Construct DNA sequences from gel-electrophoretic patterns obtained by the chain-terminator method of DNA sequencing.
- Push electrons to show the mechanisms of reactions associated with DNA replication, including chain elongation, 5'->3' exonuclease activity, 3'->5' exonuclease activity, and ligation.
Transcription and Processing
of RNA
Essential Skills
At the exam covering this chapter, you should be able to
- Explain factors that contribute to fidelity of transcription.
- Predict the effects of inducers and repressors on the lac operon.
- Push electrons to show the mechanisms of reactions associated with transcription, including chain elongation.
- Push electrons to show the mechanisms of reactions involved in post-translational processing, including capping and splicing.
- Push electrons to show the mechanism of action of beta-galactosidase, including lactose hydrolysis and conversion of lactose to 1,6-allolactose.
Synthesis of Proteins
Essential Skills
At the exam covering this chapter, you should be able to
- Explain factors that contribute to fidelity of translation.
- Find the beginnings of open reading frames (ORFs) in provided DNA sequences, and predict the sequence of mRNA and protein from the ORF sequence.
- Predict the effects of inducers and repressors on the trp operon.
- Push electrons to show the mechanisms
of action of aminoacyl-tRNA synthetases, and of chain elongation during
translation.Recombinant DNA Technology
Essential Skills
At the exam covering this chapter, you should be able to
- Explain how genes are inserted into vectors to produce recombinant DNA, and how recombinant vectors are detected.
- Explain how a DNA sequence can be specifically altered by site-directed mutagenesis.
- Compute the number of full strands, long strands, and defined strands of double-stranded DNA after each generation of polymerase chain reaction.