Graphics Gallery
Gale Rhodes
Chemistry Department
University of Southern Maine
Revised 2006/08/02
Learn how to use Swiss-PdbViewer. Work through sections 1-4 of
the Swiss-PdbViewer
Tutorial.

Topic: Amino Acids and Peptides

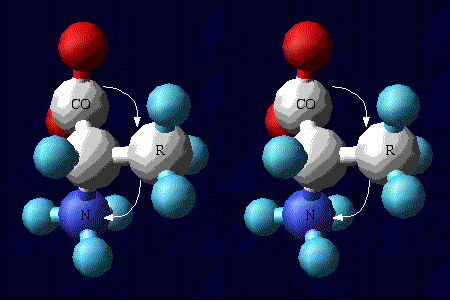
Alanine (convergent stereo
view)
It may be CO-R-Ny, but it works. With the
hydrogen of the alpha carbon pointing toward you, the amino acid
is the L enantiomer if the path from the carboxyl carbon
(CO) to the R group (R) to the amine group (N) is clockwise. If
this path is counterclockwise, the amino acid is the D
enantiomer.
Examples
1. The 20 Most Common Amino Acids
In the table below, click the name of an amino acid to view it
with Swiss-PdbViewer or your favorite graphics program. Use these
simple structures to get to know your viewer by trying out various
functions, particularly rotation, translation, and zooming.
With Swiss-PdbViewer, you can load one model after another. Then
press <control>-<tab> to turn off all but one model.
Press <control>-<tab> repeatedly to move from one model
to the next. The title bar of the grapics window tells you which
model you are viewing. The Layer Infos window shows which model is on
display, and provides display information about all models currently
loaded.
Exercises
- Confirm that each amino acid is the L-enantiomer.
- By looking at the side-chain functional groups, decide which
side chains are acidic, basic, polar but uncharged, and
nonpolar.
- The side chains of asp and glu are sometimes called
acidic side chains. Those of arg, his, and lys are called
basic. At pH 7, which of these side chains are acids, and
which are bases?
2. Oxytocin: A Nonapeptide
Oxytocin is a pituitary hormone. The structure provided here is of
oxytocin in the conformation with which it binds to its carrier
protein neurophysin (PDB file 1NPO).
(If you're interested, read
more about the neurophysin/oxytocin complex.)
Exercises
- Find the N- and C-termini of the peptide. If you find this
difficult, turn off side chains by clicking to remove checkmarks
under side in the Control Panel. To cheat, turn on labels,
but try to develop skill at recognizing structural features in the
unlabeled, stick representation that most researchers use for
exploring structure.
- Find a peptide bond. Is it cis- or trans-?
- Identify the amino acid residues in oxytocin (turn side chains
on, of course).
- Oxytocin contains a disulfide bond. Measure the dihedral angle
of atoms CB-S-S-CB. To do so, hold down <option> (right
mouse on PC) while clicking the dihedral button (#7 from left in
Tool Bar). Then click on atoms CB-S-S-CB in succession.
- Oxytocin contains a ring. What residues make the ring? What
residues contribute backbone atoms to the ring? What residues
contribute side-chain atoms to the ring? How many atoms are in the
ring?
2. Nisin: An Antibiotic
The antibiotic nisin has been used as a food preservative. It is
probably synthesized from common amino acids, but after synthesis,
chemical modifications result in unusual ring systems and some
uncommon amino acid residues. To make this exercise interesting, Deep
View does not list individual residues of nisin.
- Identify and name as many common amino acid residues as you
can.
- Draw the structures of the uncommon amino acids.
- Look carefully at the alpha carbons of each residue. Do all
residues exhibit D-configuration?
- Speculate about how common residues might have been modified
to produce the rings and other unusual aspects of nisin.

Images made with Swiss-PdbViewer
and Canvas.
Topics List
Biochemistry
Resources

