Created 2007/02/13 Under Construction
Revised 2007/03/04: DeepView project files showing key features of these molecules are complete.
Learn how to use Swiss-PdbViewer. Work through the Swiss-PdbViewer Tutorial.
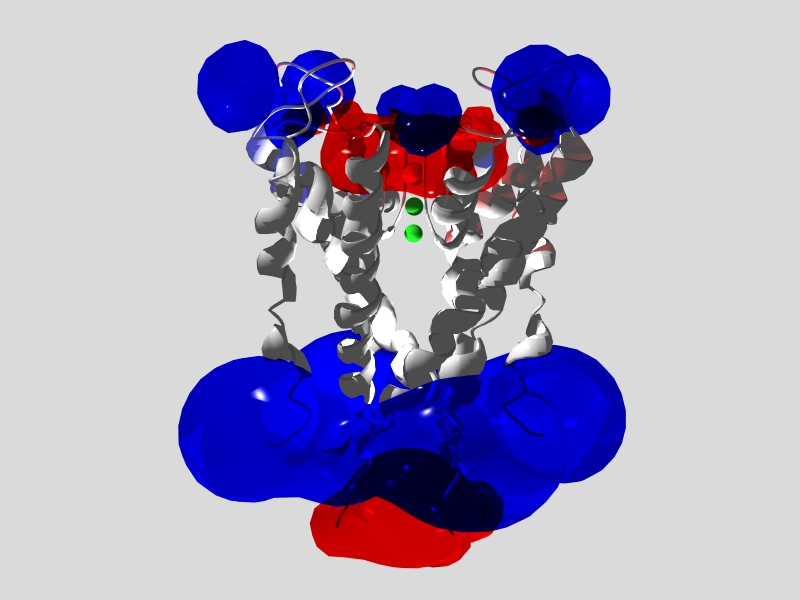
Model of potassium channel (PDB 1BL8)
with electrostatic potential surfaces.
Blue is positive potential; red is negative.
Scene prepared with DeepView and
rendered with MEGAPov.
You can obtain the models that are mentioned by file code by entering the code on the home page of the Protein Data Bank. Other specially prepared model files are linked directly to this page.
Electrostatic potentials show regions of protein surface that are electrically charged, and thus attract ions or respond to tranmembrance voltages. Electrostatic potentials are important properties of many membrane molecules and molecules that interact with ions. With DeepView, you can compute electrostatic potentials and display them as potential surfaces or use them to color the molecular surface according to potential (red = negative, blue = positive). Within the colored areas, you can see the amino-acid side chains responsible for the potentials.
To compute electrostatic potential surfaces with DeepView, use Tools:Compute Electrostatic Potential. Take the default settings, click OK, and wait for the calculation. After the calculation, close any new windows that appear, and use Wind:Electron Density and Potential Map to see the display settings. If the surface does not appear, press the cursor-up arrow to increase the displayed potential. Surfaces should appear and close around the surface (potentials are higher near the charged groups that produce them). On the map window, the sigma column shows which surface is positive and which is negative. The numbers are arbitrary units.
The pioneer model (1BL8) of a potassium channel, which gave the initial insights into how a channel can pass large ions (K+) and block small ones (Na+). Compute electrostatic potential to the see negative channel potential that attracts cations, and the positive surface potential on both sides of the membrane. Potentials show the charged regions that would respond to changes in transmembrane potential.
PDB 2BG9 is a 4.0-angstrom model of the acetylcholine receptor obtained by cryoelectron microscopy. Click HERE for a DeepView project file in which 2BG9 is superimposed onto 1I9B, a model of the water-soluble acetylcholine-binding protein, which is a structural and functional homologue of the amino-terminal ligand-binding domain of the receptor. The 1I9B model includes, as a bound ligand, an acetylcholine analog at the putative acetylcholine binding sites. In comparing the structures, note the conformational differences in the area of analog binding. A loop that is closed over the ligand in AChBP is much more open in AChR.
Calculate the electrostatic potential of either model, and you will see no dramatic basis for electrostatic attraction of acetylcholine, in great contrast to what you will see for the enzyme acetylcholinesterase, below.
Another AChBP model, 1UV6, contains a different acetylcholine analog, but with both occupied and free acetylcholine binding sites.
Click HERE for a DeepView project file with superimposed models of the enzyme with (2ACK, holo) and without (1EA5, apo) a bound competitive inhibitor at the active site. The inhibitor is edrophoium ion (3-hydroxy-N-ethyl-N,N-dimethyanilinium ion). Use DeepView to view the electrostatic potential surface of the enzyme, to see the electrical field that attracts its positively charged substrate, and make this enzyme one of nature's fastest. Also note that conformational differences between the holo and apo forms are very slight. Preorganized binding sites are usually a hallmark of enzymes with very high on-rates (rates of binding substrate).
The file contains only one chain, but the enzyme functions as a dimer, with active sites that face in opposite directions. Click HERE for a DeepView project file of the acetylcholinesterase dimer (constructed from 2ACK). Calculate the electrostatic potential of the dimer. Negatively charge groups surrounding the two active sites attract substrates from both directions. It's hard to miss this active site if you are a cation like acetylcholine.
When rhodopsin absorbs light, its covalently bound retinal molecule isomerizes from 11-cis to all trans. PDB models 1F88 (11-cis) and 2HPY(all trans) are models of these two states of the protein. Click HERE to download a DeepView project file with these models superimposed.
In each model, retinal and its covalent link to the protein, LYS296, are shown with dotted van der Waals surfaces, and their neighbors are displayed in wireframe. The cytoplasmic side of the membrane is at the C-terminal end of the model. When the file loads, the cytoplasmic side (or end) of the molecule is at the bottom.
Blink between the models to see how the retinal isomerization alters the shape of retinal, and changes the conformations of side chains in its neighborhood. The cis- to trans- change appears to open up the protein slightly. Conformational differences between the models at the C-terminal end, where G-protein would bind, are quite small, revealing little about how the isomerization of retinal alters the putative G-protein binding region.
Of course, bacteria can't see, but this model is not from a bacterium anyway; its from an archean. They can't see either, but among these models are some with both all-trans and 13-cis retinol, to show conformations of a rhodopsin before and after photoinduced retinol isomerization. This protein pumps protons across membranes, with pumping driven by conformational changes in the protein, driven in turn by photoinduced retinol isomerization.
The following file models the pumping process by showing, in sequence, crystallographic models that are thought to represent trapped intermediates in the process. Click HERE to download a DeepView project file of these models. The dotted spheres represent protons passing from carrier to carrier as they traverse the membrane from cytoplasmic side (top) to periplasmic space (bottom).
The amino acid glutamate is a prominent component of the flavor of meats and foods we think of as savory. This fifth taste category (the others are bitter, salty, sour, and sweet) is sometimes called umami, from Japanese for delicious. The receptor for this taste is a glutamate-binding GPCR that is related in an interesting way to the glutamate neurotransmitter receptors, which are called the metabotrophic glutamate receptors (types 1 and others). The taste receptor (called metabotrophic glutamate receptor type 4) has much lower affinity for glutamate, as would be appropriate for a glutamate taste sensor in comparison to a glutamate receptor in nerve transmission. The glutamate binding domain of the taste receptor appears to be a truncated form of the neurotransmitter glutamate binding domain, lacking 309 of the N-terminal residues, and as a result, possessing only 4 of the 14 nearest neighbors of bound glutamate found in the neurotransmitter type.
To see the effect of this truncation, look at PDB 1EWK, which is a model of the metabotrophic glutamate receptor type 1, one of the neurotransmitter receptors. Click HERE for a DeepView file that allows you to see the glutamate binding site with and without the first 309 residues. The first layer shows the full protein in ribbon form, with glutamate and its 4.5 anstrom neighbores (14 of them). In the second layer, to simulate the type-4 receptor, I have removed the first 309 residues, leaving again glutamate and its neighbors, now only 4 of them. It is clear that the binding affinity for glutamate would be greatly reduced in the truncated form.
So far, I am unable to find this truncated form in the human genome, although I have found what are said to be umami taste receptors. So I am having trouble verifying the description in the reading.