Graphics Gallery
Gale Rhodes
Chemistry Department
University of Southern Maine
Revised 2006/08/02
Learn how to use Swiss-PdbViewer. Work through sections 1-4 of
the Swiss-PdbViewer
Tutorial.

Topic: Citric Acid Cycle
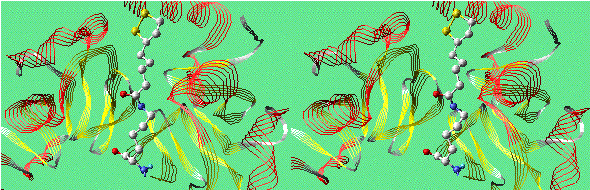
Lipoamide Arm in H Protein of Glycine Decarboxylase
(Convergent Stereo)
The long arm of lipoate-lysine, which is also found in
dihydrolipoyl transacetylase,
the E2 subunit of pyruvate dehydrogenase.

Molecules to Explore
Aconitase/Citrate Complex
Aconitase catalyzes the interconversion of citrate and isocitrate,
with enzyme-bound cis-aconitate as the intermediate. Here is a
model of a site-directed mutant (S642A) aconitase with bound
fluorocitrate, an unreactive substrate analog: 1BOK.pdb.
The fluoride atom could not be seen in the electron density, so it
was modeled as citrate.
Citrate binds to one iron atom of the Fe4S4
cluster at the active site of aconitase. This iron atom has six
ligands: three sulfurs in the cluster, oxygen of the C-3 OH group of
citrate, one oxygen of the carboxy group on C-3 of citrate, and a
water molecule.
C-3 of citrate is not chiral, because it carries two identical
carboxymethyl groups, one derived from oxaloacetate, the other from
acetyl-CoA. Aconitase distinguishes between these two seemingly
identical groups. In the product, isocitrate, the OH group is on the
carbon derived from oxaloacetate, not from acetyl-CoA. The following
exercises will help you to see how the enzyme accomplishes this
conversion.
Think About It
- Restrict your view to atoms within 4 or 5 angstroms of
flurocitrate (FLC756), including the iron-sulfur cluster (FS4757).
Find the iron atom that binds citrate and measure the distance to
each of its six ligands.
- In addition to the iron atom, what residues bind citrate?
- What additional non-cluster ligand is present on the same iron
atom that is bound to citrate? This ligand is one of the
substrates of the aconitase reaction.
- Arrange the view so that you see C-3 of citrate as in a Fisher
projection, with the C-3 hydroxyl pointing left and toward you,
and the carboxyl on C-3 pointing right and toward you. You will be
looking at citrate through the FeS cluster. Above and below C-3
are two carboxymethyl groups. The upper one is derived from
acetyl-CoA, the lower one from oxaloacetate.
- Notice that a water molecule (answer to question 3 above) lies
above the lower CH2 group, the one derived from
oxaloacetate. The CH2 group derived from acetyl-CoA is
far away from this water molecule. In the product complex with
isocitrate, this water becomes the new OH group on C-2, and
the C-3 OH of citrate becomes an Fe-bound water molecule. You
might imagine that citrate could bind "upside down" from this
orientation, allowing the other CH2 to be the OH
acceptor , but note that ARG452, on your right, binds the C-3
carboxyl of citrate. The only way citrate can bind is in the
orientation shown in this model, so the CH2 group
derived from acetyl-CoA cannot be the acceptor of the new OH
group.
Malate Dehydrogenase/Malate /NAD+ Complex
Malate dehydrogenase (MDH) catalyzes the reversible oxidation of
L-malate to oxalacetate. Click here to download a model of the
E. coli MDH with bound NAD+ and malate: 1CME.pdb.
Because this complex is catalytically active, it is not possible
to determine its structure by crystallography. 1CME is a theoretical
model built from a crystallographic model of MDH bound to citrate,
which binds in similar fashion to malate. The investigators removed
the citrate coordinates from the file, and built a model of
NAD+ into the its binding site, based on its position in
crystallographic models of MDH/NAD+ complexes. Then they
built a malate model into its presumed binding site, based on
interactions observed for citrate.
Think About It
- Display the model as a backbone model. Select residues 1-144
and color them green. Select residues 145-312 and color them
yellow. Then display malate and NAD+ as space-filling
models. MDH has two domains. Domain I binds NAD+, and
domain II provides the catalytic residues HIS177 and ASP150. Both
domains are involved in binding malate.
- Restrict your view to malate and NAD+. What is the
distance between C-2 of malate and C-4 of NAD+? During
catalysis, a hydride ion moves between these two carbons.
- Add atoms within 6 or 7 angstroms of malate to the view. What
amino acids are involved in binding the carboxyls of malate? Which
are from domain I and which from domain II?
- Residues HIS177 and ASP150 are essential to catalysis. Add
these side chains to the view, and measure the distances between
interacting atoms in HIS, ASP, and malate. Note the resemblance of
these three groups to the catalytic HIS, ASP, and SER of serine
proteases. The position of the C-2 OH of malate is analogous to
that of the side-chain OH of SER in serine proteases.
- C-2 of L-malate is chiral. Is its configuration
R or S? Remember that there is a hydrogen atom at
C-2 that is not shown.
- Imagine that the D-enantiomer of malate were bound at
this site, with the carboxyls bound as shown in this model. This
would mean that positions of the C-2 OH and the C-2 H atom (not
shown) would be swapped. Why can MDH not transfer hydride between
NAD and D-malate?
Using SwissPdbViewer, you can see what it's like to try to place a
substrate model into the active site. Make sure that Netscape is
using SwissPdbViewer for files of MIME type chemical/x-pdb. Then
download these two files:
- MDH.pdb: a model of
MDH/NAD+ without malate.
- Malate.pdb: a model of malate,
in the correct conformation for binding.
With these two files loaded into SPdbV, try to place the malate
model into the active site of the enzyme. You can move models
separately in SPdbV by use of the Control Panel. Each model has a
can move button. Click to remove the checkmark from the can
move box, and that model will remain motionless while you move
other models. It helps to display surface dots on the malate model
while trying to fit it into the active site.
Once you have fitted the malate into place, load 1CME.pdb into
SPdbV and use Tools: Magic Fit to superimpose 1CME onto MDH.
Be sure that MDH is the reference, so that it does not move during
the superposition. After superposition, center on malate in 1CME and
compare its position to the current position of your malate molecule
in the Malate.pdb layer.

Topics List
Biochemistry
Resources


![]()