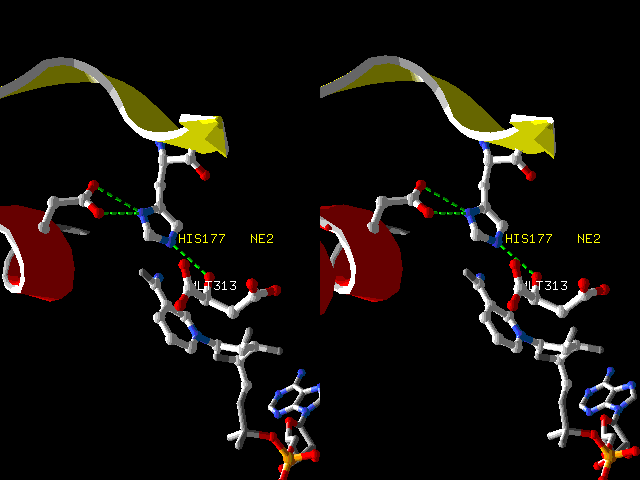Get To Know Histidine
A versatile, important, and confusing amino acid.
Gale Rhodes
Chemistry Department
University of Southern Maine
|
|
The Active Site of Malate Dehydrogenase
(1cme.pdb).
In the conversion of malate to
oxaloacetate, it is proposed that histidine acts as a
general base, abstracting the proton from the hydroxyl
of malate (MLT313), and driving the transfer of a
hydride ion (not shown) onto the nicotinamide ring of
NAD+ (below malate). If the proposal is
correct, then before the proton transfer, the
histidine ring is in the neutral imidazole form,
tautomer C in the figure below. Proton transfer
converts the ring to the imidazolium ion (resonance
hybrid B).
Convergent
stereo image, prepared with DeepView.
|
Physiologically
IMPORTANT
Ionization States of the Histidine Side Chain
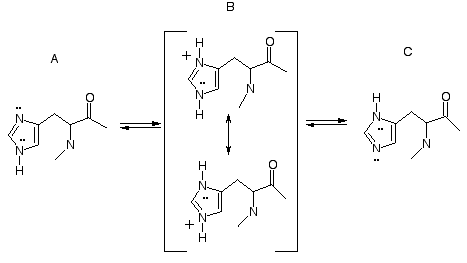
Ionic and Tautomeric States of the Histidine
Side Chain
The side chain of histidine includes the ionizable
imidazole ring. The pKa value for the ring is
approximately 7.0, so at physiological pH, both the acid and base
forms are present. The acid form, the imidazolium ion B, is a
resonance hybrid of two practically equivalent contributing forms
(remember that these two contributors represent one
structure). Either of the two ring nitrogens can release a proton
(H+) to produce the conjugate base form, imidazole
(A or C). Release of proton from the upper nitrogen of B produces
the imidazole tautomer A, and release of proton from the lower
nitrogen produces imidazole tautomer C. The tautomers equilibrate by
way of the protonated form B. At pH values near 7, all three forms,
A, B, and C, are present in equilibrium.
Because the acid form, imidazolium B, is a hybrid
of the two contributors, it is plausible to show release of a proton
from either nitrogen atom in proposing a reaction mechanism involving
the imidazolium form as a general acid. Because the base form,
imidazole, exists as two equilibrating tautomers A and C, it is
plausible to write either tautomer in proposing a reaction mechanism
involving the imidazole form as a general base.
A Physiologically
RARE
Ionization State of Histidine
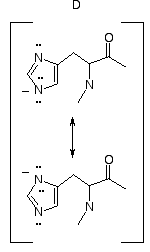
The Imidazolate Ion
Abstraction of a proton from imidazole (A or C)
results in the imidazolate ion D, a resonance hybrid of two
practically equivalent contributors. The pKa of imidazole
itself is 14.58*, so the pKa of imidazole in a histidine
residue of a protein should be near this value. Thus this ionization,
producing a free imidazolate ion, would not occur under physiological
conditions. In proposing a reaction mechanism for a physioligical
process, it is not plausible to propose loss of a proton from an
imidazole tautomer (A or C) to form a free imidazolate ion D. Any
proposal of free imidazolate ion as an intermediate in an enzymatic
reaction is simply incorrect.
Imidazolate ion can be produced in organic
solvents by the action of strong irreversible bases, such as hydride
ion. The ion can also serve as a ligand in transition-metal
complexes.
Thanks to Professor Daryl Eggers of San Jose State
University for pointing out to me that imidazolate ion makes at least
one appearance in biology, in the histidine side chain that bridges
copper and zinc ions in the enzyme copper-zinc superoxide dismutase.
Mutations in this enzyme can lead to amyotrophic lateral sclerosis
(ALS), also known as Lou Gehrig's disease. The bridging histidine is
shown in the illustration below.
|
|
|
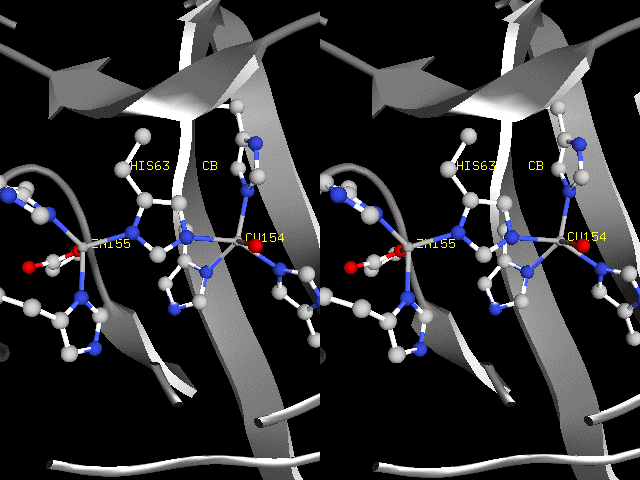
Imidazolate Ion in a Biological Setting
Metal-bridging imidazolate in human Cu-Zn superoxide
dismutase (1hl5.pdb).
The side chain of histidine 63 appears to be an imidazolate
ion, in which both protons of
imidazolium ion are replaced by strong Lewis acids, copper
(I or II) and zinc (II) ions.
|
*The Chemistry of Heterocycles : Structure,
Reactions, Syntheses, and Applications, 2nd edition, Theophil
Eicher and Siegfried Hauptmann, New York: John Wiley and Sons, 2003,
page 166.
Back to Goodies List
Back to Biochemistry
Resources
HOME