Revised 2008/05/22
Review the basics of reaction kinetics in your general or organic chemistry textbook. Make sure that you know the meaning of order and molecularity in chemical reactions, and review the rate equations for first- and second-order reactions.
BEFORE trying to read the entire chapter on enzymes, learn to derive the Michaelis-Menten equation from this basic model of enzyme action (sometimes called the Michaelis-Menten model):
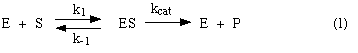
By learning this derivation, you will get some good practice at manipulating important relationships in enzyme kinetics, and you will reinforce your understanding of parameters like Vmax, kcat, and Km, which allow you to compare the efficiencies of various enzymes.
After you learn this derivation, you will find the chapter much easier to read.
For a clear, step-by-step derivation of this equation, click here.
(At first class on this topic)
From the Michaelis-Menten model [equation (1) above], derive the Michaelis-Menten equation. Show all algebraic manipulations, and define all terms where they first appear.