Enzyme Kinetics: Answers to Problems
Gale Rhodes
Department of Chemistry
University of Southern Maine
Portland, Maine 04104-9300
Revised 2006/07/14
Enyzme Kinetics and Inhibition: Example for Analysis in
Class
(Data taken from Lindquist, R.N., Problems and Solutions Guide to
Accompany Rawn's Biochemistry, Neil Patterson Publishers, 1990.)
The table gives enzyme-catalyzed reaction rates (initial rate,
V0) measured at various substrate concentrations in
solutions with [E] = 1.2 x 10-4 mmol/L.
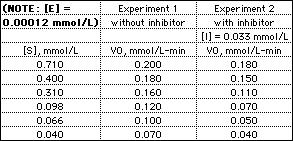
- Use the data from Experiment 1 to calculate
Vmax, Km, and kcat for this
enzyme-catalyzed reaction.
- Use the data from Experiment 2 to determine the apparent
Vmax and Km in the presence of the
inhibitor. From this information, determine the type of inhibition
(competitive, noncompetitive or uncompetitive) and calculate the
dissociation constant KI for the inhibitor. For help,
see Summary of
Kinetic Effects of Reversible Inhibitors.
Answers
Results obtained by Lineweaver-Burke plot and linear least squares
line fitting with Excel ("trendline, linear"); if you graph by hand
or use other programs, your answers may differ slightly. Units of all
quantities in calculations are shown in brackets.
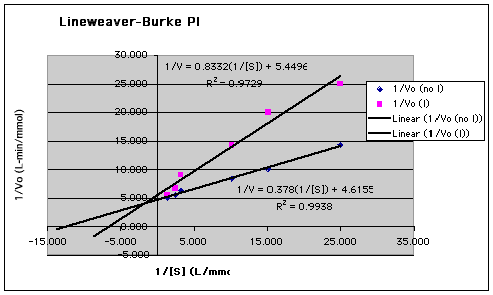
Sorry about the chopped legends. Excel excelling as
usual.
Experiment 1:
- Vmax is the inverse of the (1/V0)
-intercept on the Lineweaver-Burke plot:
Vmax = 1/(4.6155 [L-min/mmol S]) = 0.217
mmol S/L-min
- The slope of the Lineweaver-Burke plot is equal to
Km/Vmax, so Km = slope x
Vmax:
Km = (0.378 [L-min/mmol S]/[L/mmol
S]) x (0.217 [mmol S/L-min]) = 0.0820 mmol S/L
- Vmax = kcat x
[E]total so kcat =
Vmax/[E]total
kcat = (0.217 [mmol S/L-min]/(1.2 x 10-4
[mmol E/L] = 1.81 x 103 [(mmol S/mmol
E)/min] x (1 min/60 sec)
kcat = 30.1 sec-1
Experiment 2:
Using the same methods as in experiment 1,
- Vmax(apparent) = 0.183 mmol S/L.min
- Km(apparent) = 0.152 mmol S/L
Inhibition is competitive, but note that with realistic data like
this, there is some uncertainty in this conclusion. I conclude that inhibition
is competitive because the inhibitor raises Km by
almost 85% and lowers Vmax by only 16%. Given the poor data (look
at the R2 values), the change is Vmax is probably not significant.
You may judge otherwise.
- From the Summary
of Kinetic Effects of Reversible Inhibitors, for
competitive inhibition,
KI =
Km[I]/(Kmapp -
Km)
KI = (0.0820 [mmol S/L] x 0.033 [mmol
I/L])/(0.152 [mmol S/L] - 0.0820 [mmol
S/L])
KI = 0.039 mM
Compare KI with Km (not
Kmapp). The inhibitor binds about twice as
tightly as the substrate.
Goodies
Biochemistry
Resources


